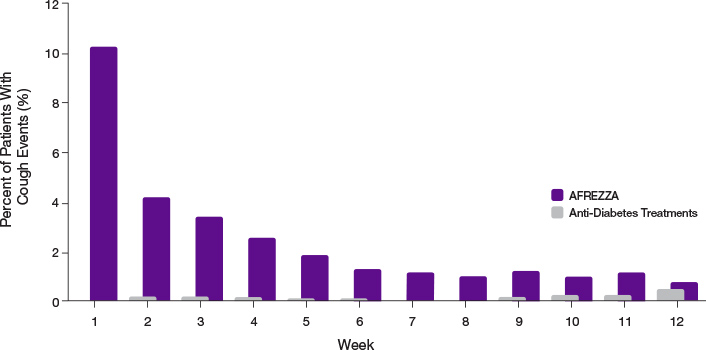
- 93.2% of cough episodes were characterized as intermittent or single defined episodes3




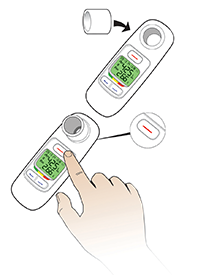
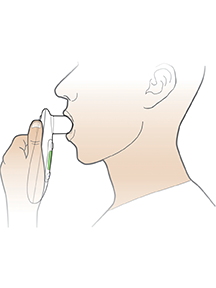
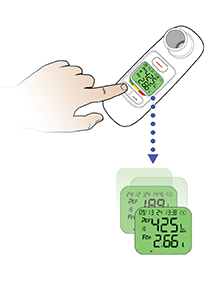
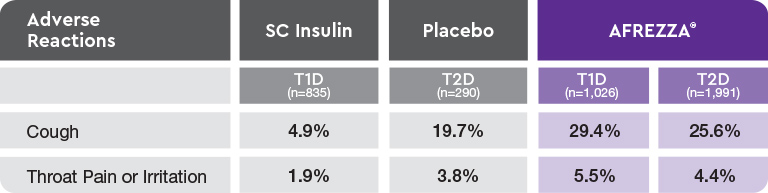
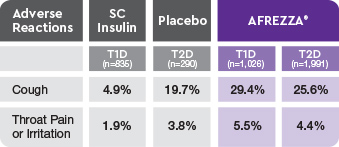
AE=adverse event; FEV1=forced expiratory volume in 1 second; T1D=type 1 diabetes; T2D=type 2 diabetes.
References: 1. Afrezza (insulin human) Inhalation Powder Prescribing Information. MannKind Corporation. 2. Data on file. MannKind Corporation. 3. McGill JB, Peters A, Buse JB, et al. Comprehensive pulmonary safety review of inhaled Technosphere® insulin in patients with diabetes mellitus. Clin Drug Investig. 2020;40(10):973-983. 4. Oches M, O’Brodovich H. 5 – The Structural and Physiologic Basis of Respiratory Disease, In: Kendig & Chernick’s Disorders of the Respiratory Tract in Children. 8 ed. W.B. Saunders; 2012: 35-74 5. Raskin P, Heller S, Honka M, et al. Pulmonary function over 2 years in diabetic patients treated with prandial inhaled Technosphere Insulin or usual antidiabetes treatment: a randomized trial. Diabet Obes Metab. 2012;14(2):163-173. 6. Levin PA, Heinemann L, Boss A, Rosenblit PD. Impact of symptomatic upper respiratory tract infections on insulin absorption and action of Technosphere inhaled insulin. BMJ Open Diabetes Res Care. 2016;4(1):e000228. 7. Sonmol PEF. User manual. 2023.
© MannKind Corporation October, 2025. US-AFR-2749
Afrezza® (insulin human) Inhalation Powder is a rapid acting inhaled human insulin indicated to improve glycemic control in adult patients with diabetes mellitus.
Limitations of Use: Not recommended for the treatment of diabetic ketoacidosis, not recommended in patients who smoke or have recently stopped smoking.
WARNING: RISK OF ACUTE BRONCHOSPASM IN PATIENTS WITH CHRONIC LUNG DISEASE. Acute bronchospasm has been observed in AFREZZA-treated patients with asthma and Chronic Obstructive Pulmonary Disease (COPD). AFREZZA is contraindicated in patients with chronic lung disease such as asthma or COPD. Before initiating AFREZZA, perform a detailed medical history, physical examination, and spirometry (FEV1) to identify potential lung disease in all patients.