physiologic insulin
ultra-rapid delivery1,2
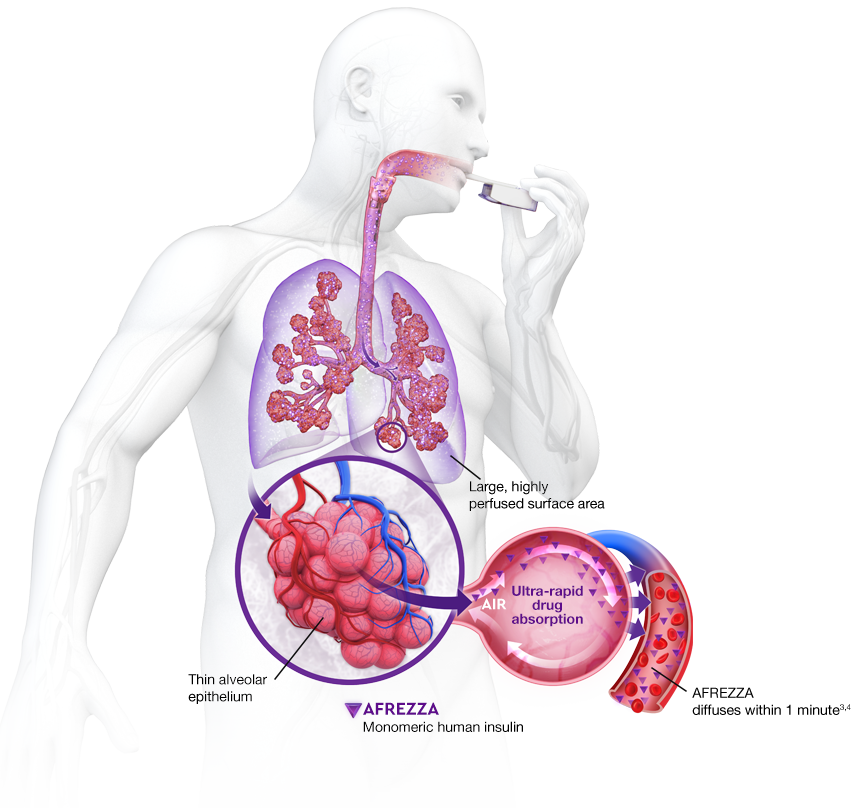
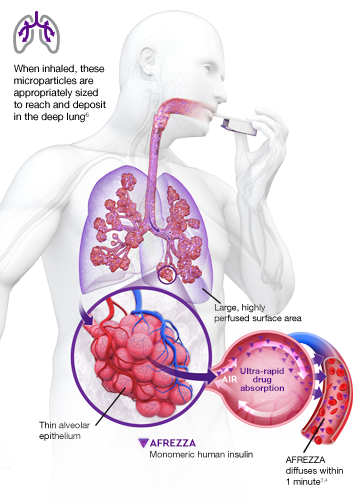


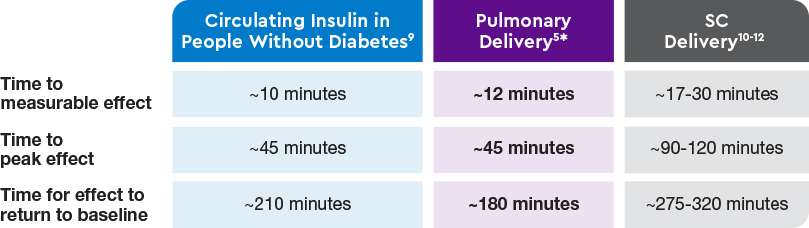
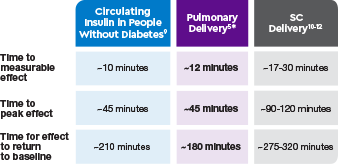
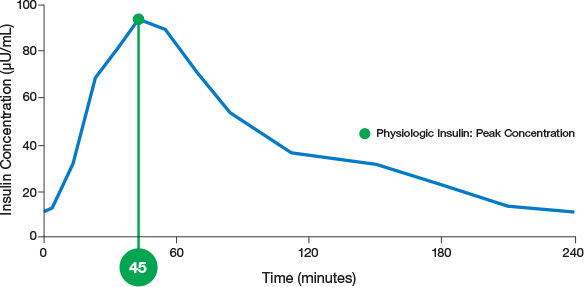
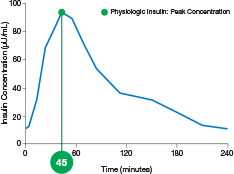
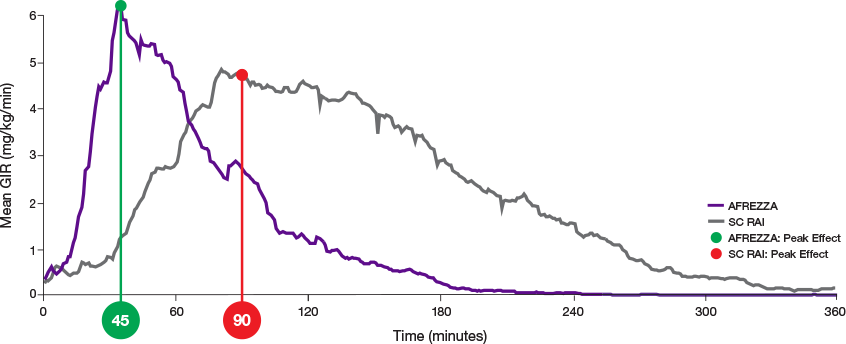
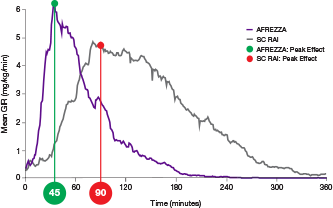
AUC=area under curve; GIR=glucose infusion rate; MGTT=mean glucose tolerance test; PK=pharmacokinetics; PD=pharmacodynamics; RAI=rapid-acting insulin; SC RAI=subcutaneous rapid-acting insulin; T1D=type 1 diabetes.
References: 1. Heinemann L, Baughman R, Boss A, Hompesch M. Pharmacokinetic and pharmacodynamic properties of a novel inhaled insulin. J Diabetes Sci Technol. 2017;11(1):148-156. 2. Heinemann L, Parkin CG. Rethinking the viability and utility of inhaled insulin in clinical practice. J Diabetes Res. 2018:4568903. doi: 10.1155/2018/45689 3. Data on file. MannKind Corporation. 4. Grant M, Harris E, Leone-Bay A, Rousseau K. Poster presented at: Diabetes Technology Society Meeting; November 2-4, 2006; Atlanta, GA. 5. Afrezza (insulin human) Inhalation Powder Prescribing Information. MannKind Corporation. 6. Leone-Bay A, Baughman R, Smutney C, Kocinsky J. Innovation in drug delivery by inhalation. OnDrugDelivery Magazine. 2010;4-8. 7. Sarala N, Bengalorkar G, Bhuvana K. Technosphere: new drug delivery system for inhaled insulin. Fut Prescriber. 2012;13:14-16. https://doi.org/10.1002/fps.90 8. Rave K, Heise T, Heinemann L, Boss AH. Inhaled Technosphere insulin in comparison to subcutaneous regular human insulin: time action profile and variability in subjects with type 2 diabetes. J Diabetes Sci Technol. 2008;2(2):205-212. 9. Caumo A, Bergman RN, Cobelli C. Insulin sensitivity from meal tolerance tests in normal subjects: a minimal model index. J Clin Endocrinol Metab. 2000;85(11):4396-4402 10. Grant M, Heise T, Baughman R. Comparison of pharmacokinetics and pharmacodynamics of inhaled technosphere insulin and subcutaneous insulin Lispro in the treatment of type 1 diabetes mellitus. Clin Pharmacokinet. 2022;61(3):413-422. doi:10.1007/s40262-021-01084-0 11. Fiasp (insulin aspart injection) Prescribing Information. Novo Nordisk. 12. Lyumjev (insulin lispro-aabc injection) Prescribing Information. Eli Lilly and Company.
© MannKind Corporation October, 2025. US-AFR-2748
Afrezza® (insulin human) Inhalation Powder is a rapid acting inhaled human insulin indicated to improve glycemic control in adult patients with diabetes mellitus.
Limitations of Use: Not recommended for the treatment of diabetic ketoacidosis, not recommended in patients who smoke or have recently stopped smoking.
WARNING: RISK OF ACUTE BRONCHOSPASM IN PATIENTS WITH CHRONIC LUNG DISEASE. Acute bronchospasm has been observed in AFREZZA-treated patients with asthma and Chronic Obstructive Pulmonary Disease (COPD). AFREZZA is contraindicated in patients with chronic lung disease such as asthma or COPD. Before initiating AFREZZA, perform a detailed medical history, physical examination, and spirometry (FEV1) to identify potential lung disease in all patients.