INHALE-3 Study: Comparing AFREZZA to injectables and pumps
Learn about our latest INHALE-3 clinical trial

Dr. Kevin Kaiserman
SVP Medical Affairs

INHALE-3 Study Summary
Primary endpoint met: AFREZZA demonstrated similar A1C control compared with Usual Care1

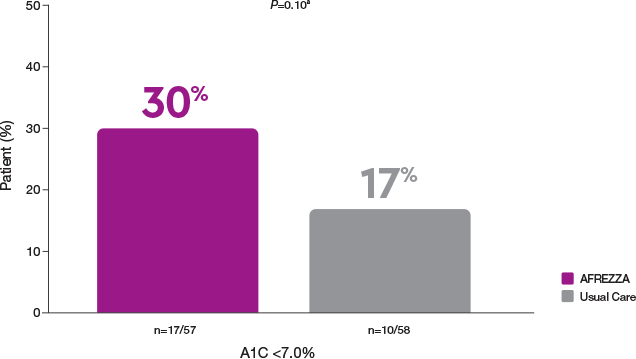
More patients achieved A1C goal vs MDI1
30% of participants who switched from MDI to AFREZZA achieved an A1C <7% compared to 4% who remained on MDI
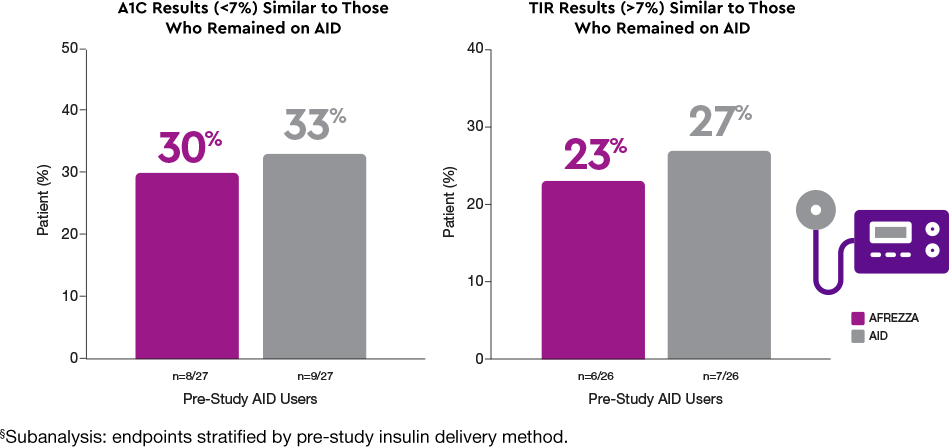
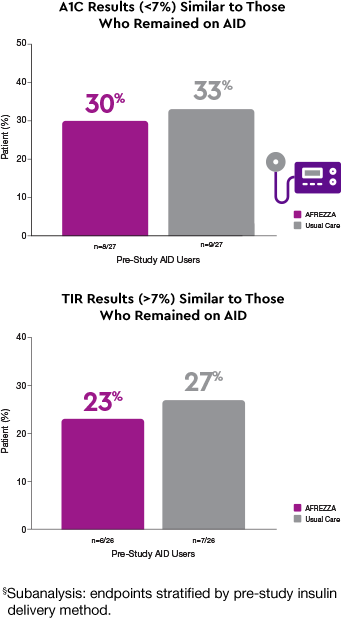
Achieved similar results to AID without a tethered device1
The AFREZZA and AID groups achieved comparable glycemic goals (A1C <7% and TIR >70%)
Demonstrated established safety profile1
- Similar percentage of hypoglycemia by CGM
- No significant difference in FEV1
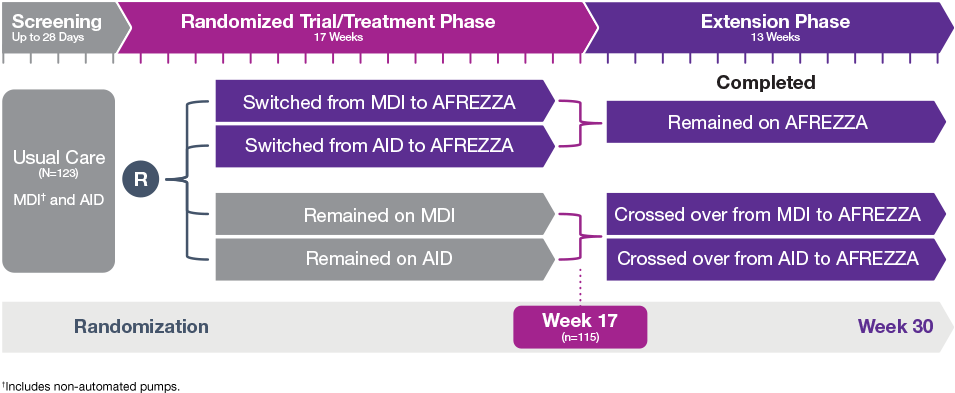
Study Design
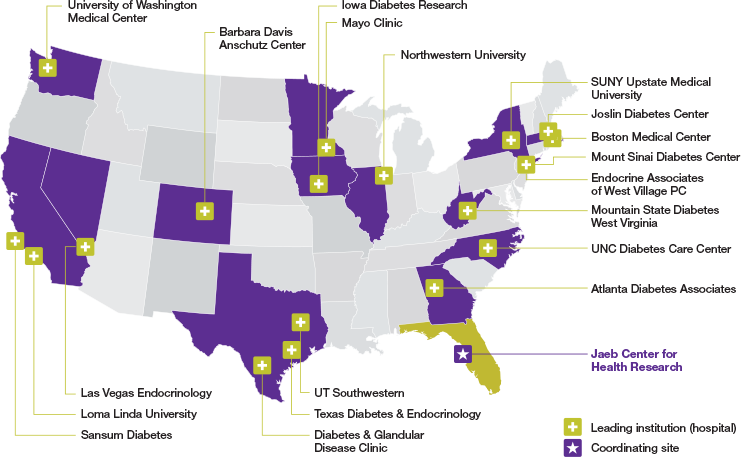
Diabetes knows no boundaries: 19 sites selected across the U.S.
123 randomized participants ≥18 years old with T1D1,2
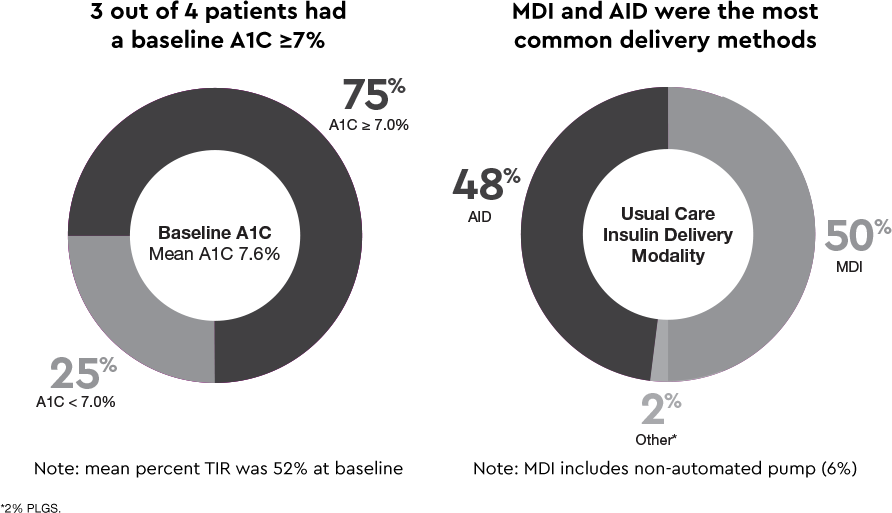
INHALE-3 patient characteristics1,2
INHALE-3 study design1,2
Primary Endpoint Data
At baseline:
- Both the AFREZZA group (n=62) and the usual care group (n=61) had a baseline average A1C of 7.6%, indicating similar baseline glycemic control in both groups
After 17 weeks:
- The AFREZZA group (n=57) maintained an average A1C of 7.6%
- The Usual Care group (n=58) had an average A1C of 7.5%
- The non-inferiority margin of 0.4% was met (P=0.01‡)
‡17-week treatment difference between AFREZZA and Usual Care (95% CI): 0.11 (-0.10, 0.33)
Patients randomized to AFREZZA maintained A1C levels of 7.6 over 17 weeks2
Efficacy
AFREZZA vs Usual Care: more patients on AFREZZA achieved A1C goals1
More AFREZZA patients achieved an A1C <7% compared to Usual Care
A1C Change From Baseline in All Participants
(Exploratory endpoint)
ª17-week treatment difference between AFREZZA and Usual Care (95% CI); P-value.
- Proportion of patients experiencing a >0.5% change in A1C: Among all subjects, 21% of AFREZZA patients had a >0.5% decrease in A1C compared with 5% in the Usual Care group. Conversely, 26% of AFREZZA patients achieved a >0.5% increase in A1C, compared with 3% in the Usual Care group
In patients with a baseline A1C >7%, only AFREZZA patients achieved A1C goals by Week 17
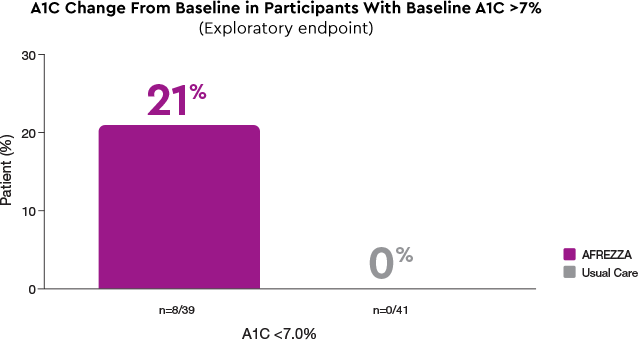
- Proportion of patients experiencing a >0.5% change in A1C: Among subjects with a baseline A1C >7%, 28% of AFREZZA patients had a >0.5% decrease in A1C compared with 7% in the Usual Care group. Conversely, 21% of AFREZZA patients achieved a >0.5% increase in A1C, compared with 2% in the Usual Care group
AFREZZA vs Usual Care: time in range outcomes1
AFREZZA maintained TIR at Week 17
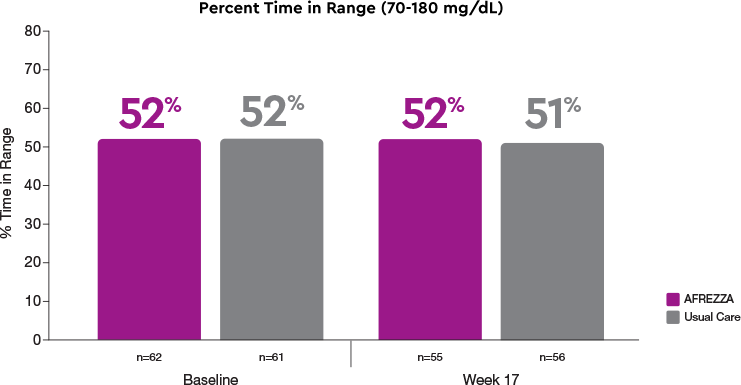
More AFREZZA patients improved TIR
Time in Range (70-180 mg/dL) >70% at 17 Weeks
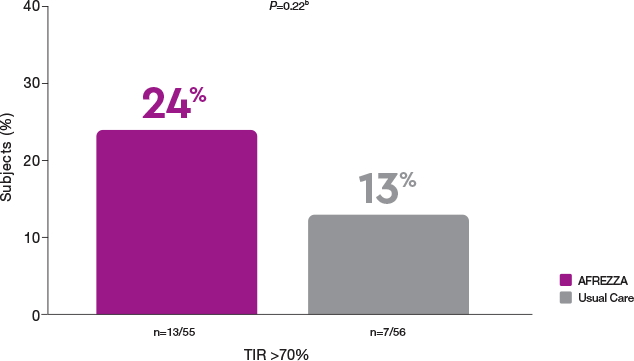
b17-week treatment difference between AFREZZA and Usual Care (95% CI); P-value.
- Proportion of patients experiencing a ≥10% change in TIR: Among all subjects, 25% of AFREZZA patients increased TIR by ≥10% compared to 14% on Usual Care. Conversely, 31% of AFREZZA patients decreased TIR by ≥10%, compared to 19% on Usual Care
Delivery methods matter1§
More AFREZZA patients achieved…
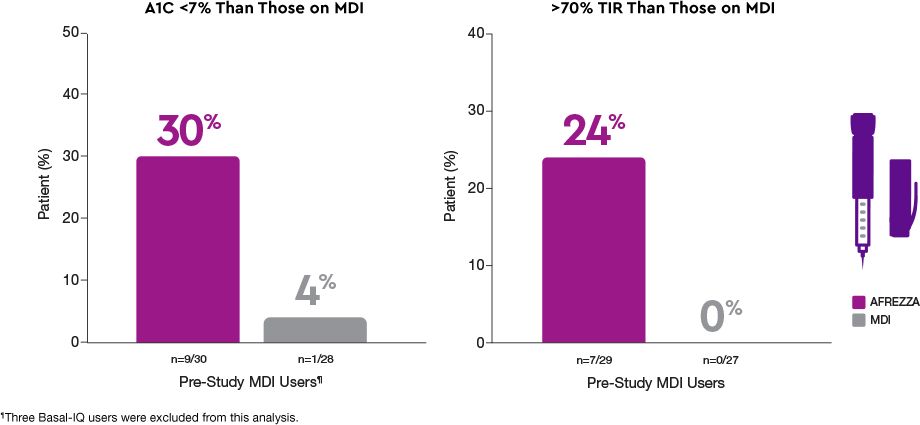
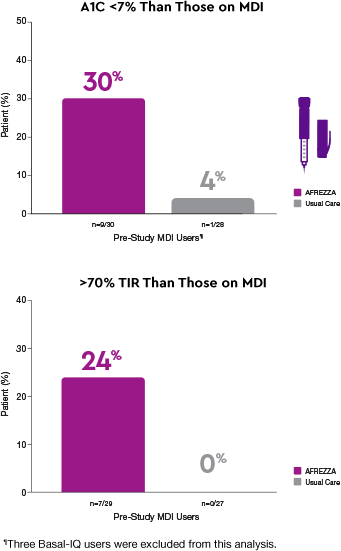
Patients who switched to AFREZZA experienced…
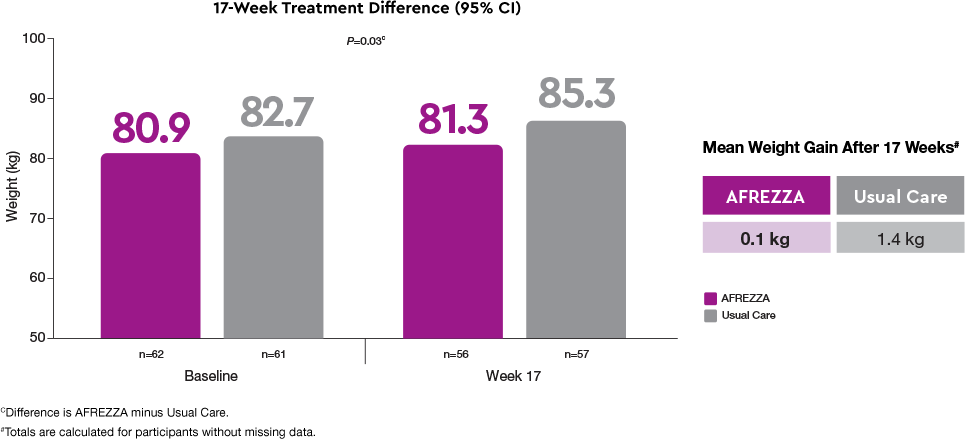
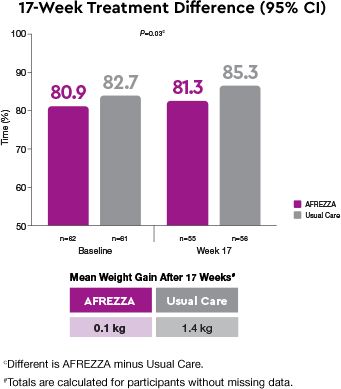
Weight-neutrality1
The Usual Care group gained statistically more weight than the AFREZZA group
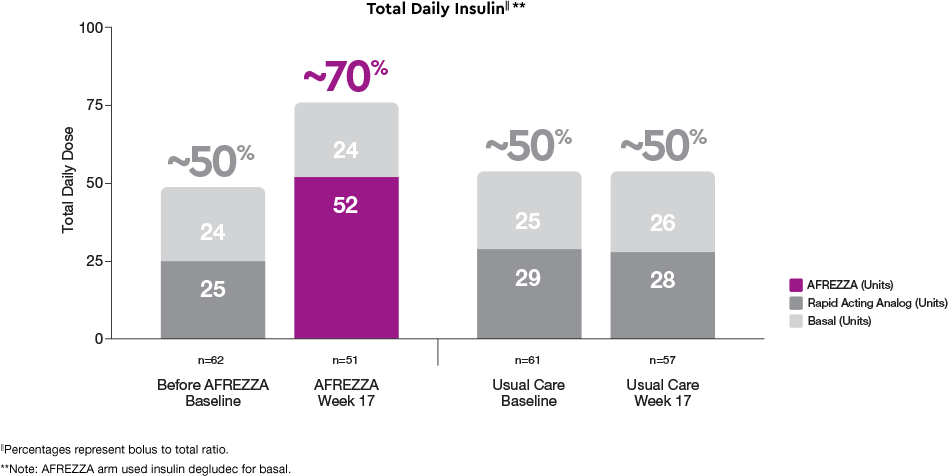
AFREZZA UNITS: different bolus to basal insulin dose ratio at Week 171
Before AFREZZA (baseline): Patients on usual care (which includes rapid-acting analog bolus insulin) had a ~50/50 bolus-to-basal insulin ratio
AFREZZA at Week 17: Titrated dose was ~70% bolus (in AFREZZA UNITS) to 30% basal insulin
Usual Care baseline and Usual Care Week 17: For those continuing on usual care, the ratio remains consistent at 50% bolus and 50% basal
Safety
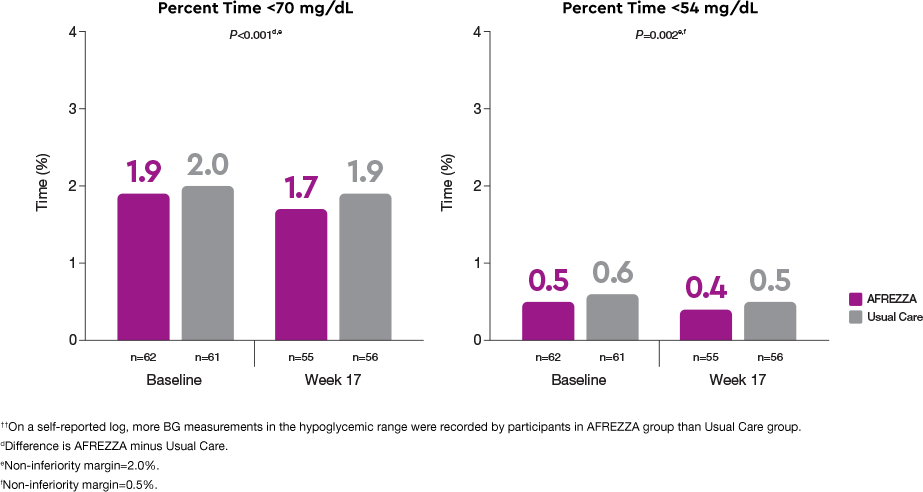
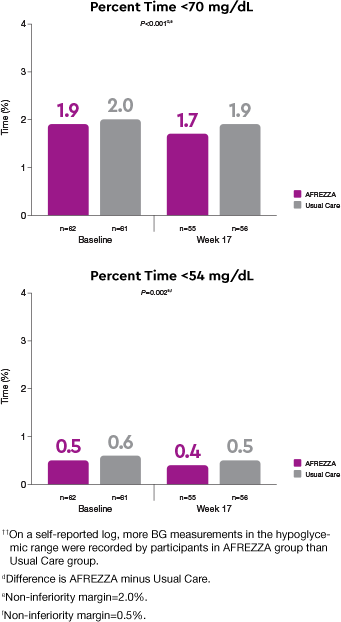
Safety: comparable time in hypoglycemic range observed1††
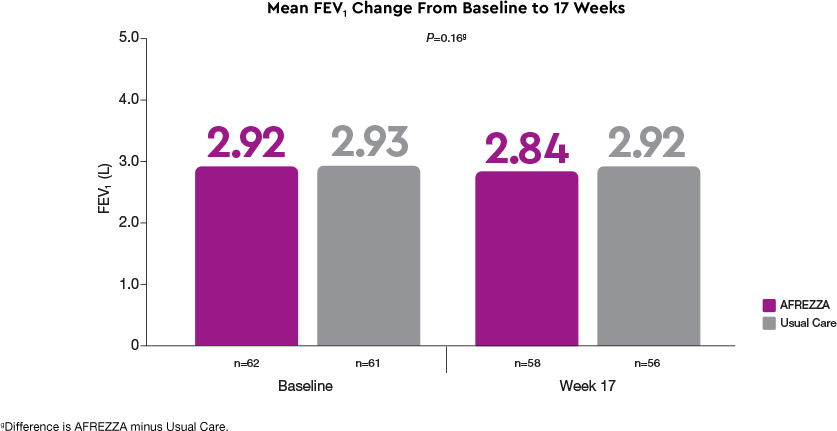
No significant change in pulmonary function1
FEV1 showed no significant difference between groups at 17 weeks
Adverse events1‡‡
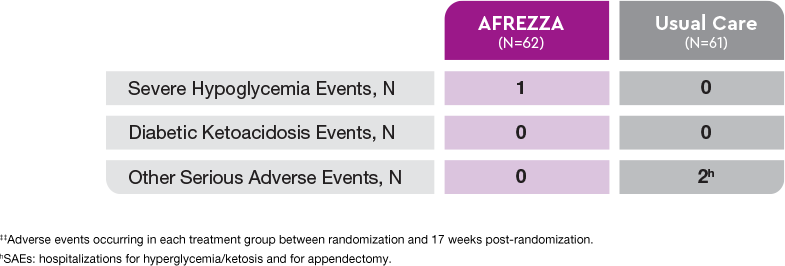
Treatment-emergent adverse events:
- Cough is the most commonly reported AE in the AFREZZA group (14 of 62)
- Only other TEAE >5% was shortness of breath (5 of 62)
Individualized Insulin Therapy

Individualized insulin therapy for your patient: a possible scenario based on INHALE-3 study design and results1,3
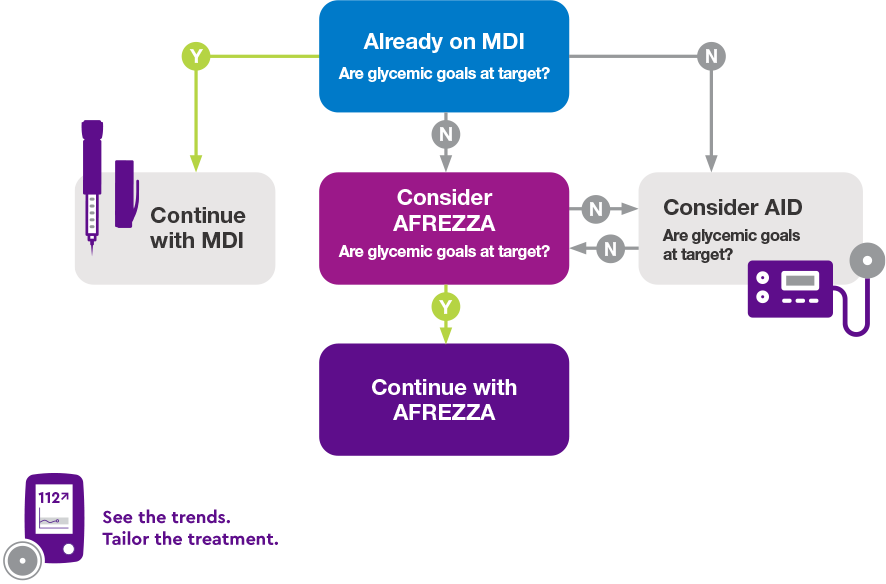
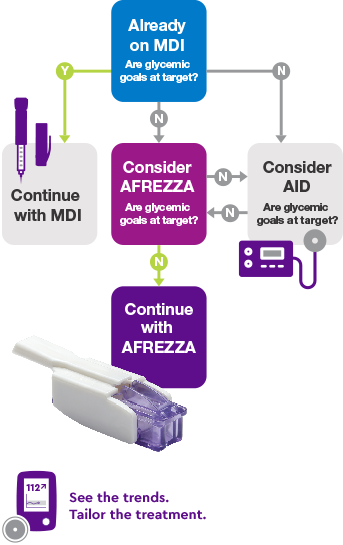
A1C=glycated hemoglobin; AID=automated insulin delivery; BG=blood glucose; CGM=continuous glucose monitor; FEV1=forced expiratory volume in one second; MDI=multiple daily injections; PLG=predictive low-glucose suspend technology; SC RAI=subcutaneous rapid-acting insulin; TIR=time in range; T1D=type 1 diabetes.
References: 1. Data on File (INHALE-3 Clinical Study Report 2024). MannKind Corporation. 2. ClinicalTrials.gov identifier: NCT05904743. 3. American Diabetes Association Professional Practice Committee. Diabetes Care. 2024; 47 (Supplement_1): S158-S178.
© MannKind Corporation October, 2025. US-AFR-2753
